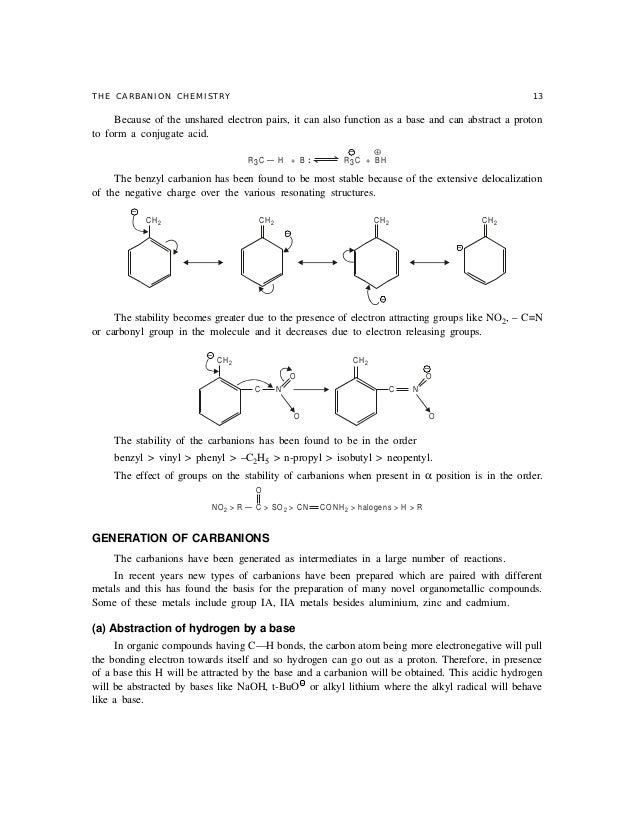
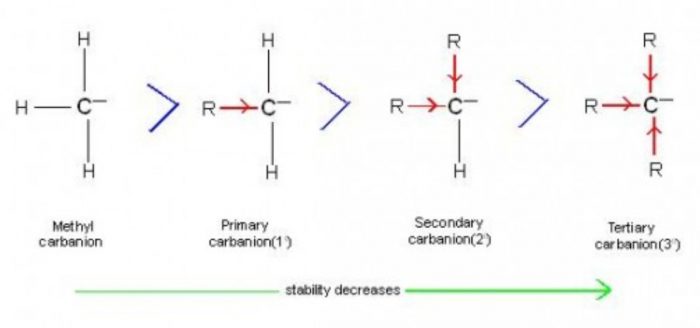
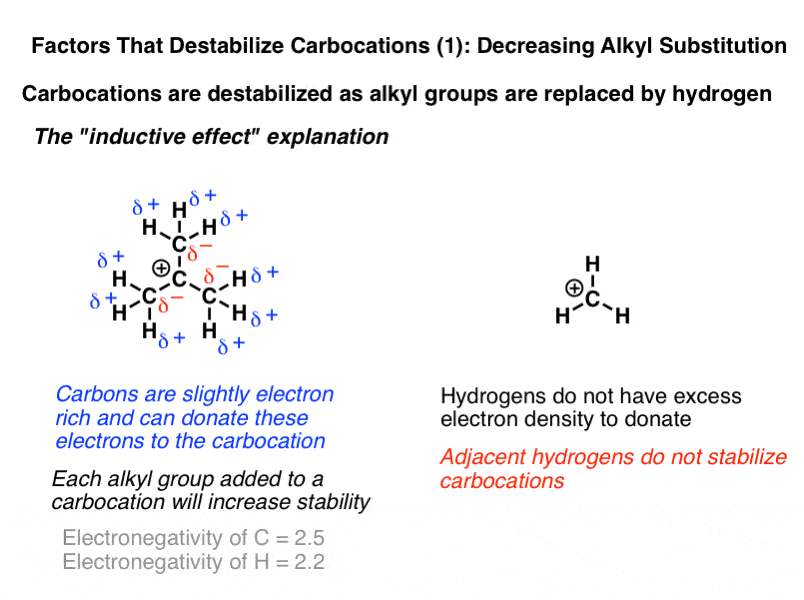
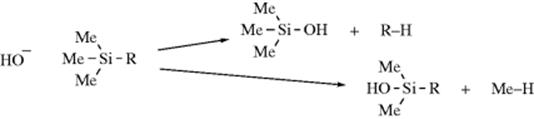
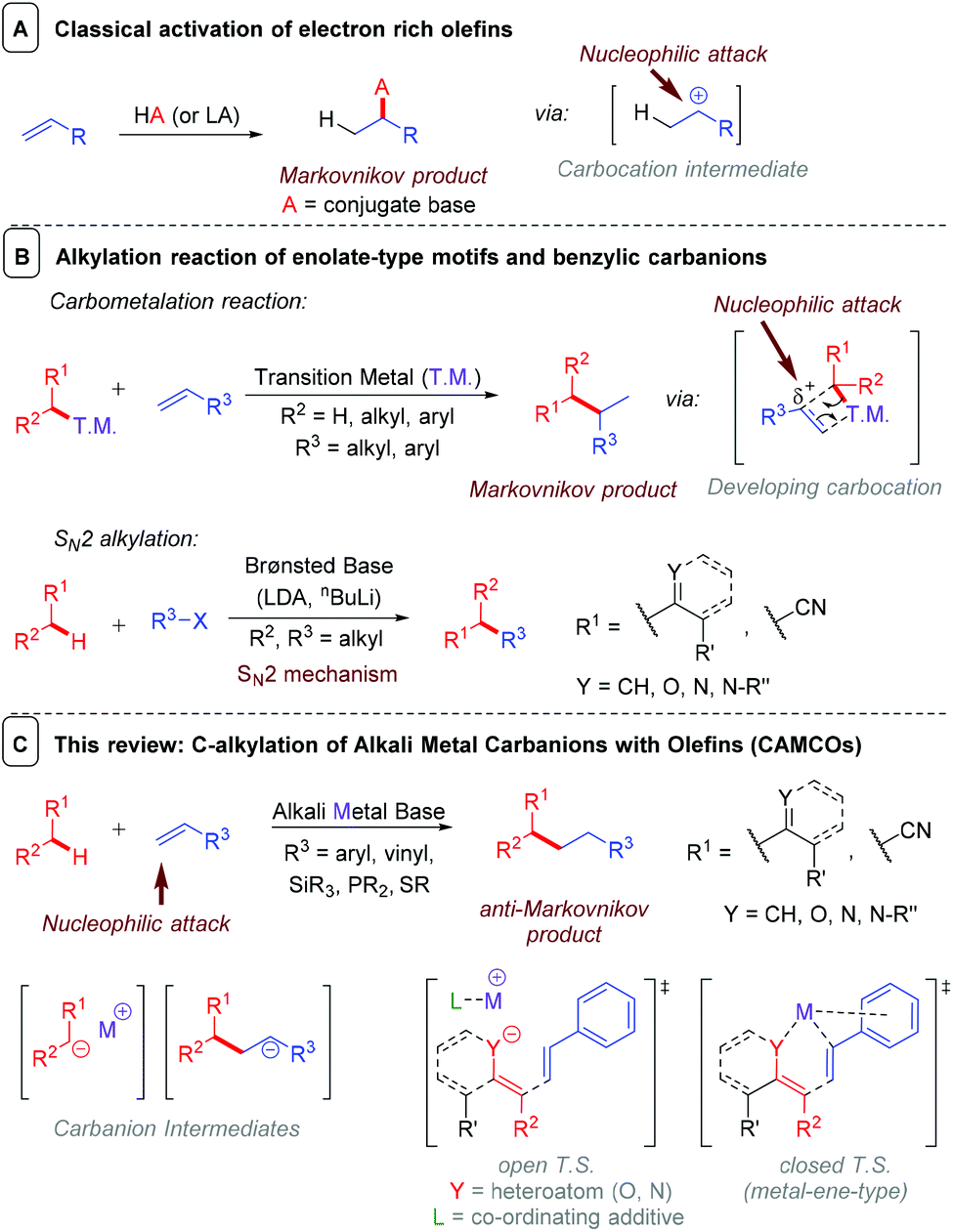
Stability order of carbanions decreases as we move from primary to tertiary anion because due to i effect of methyl groups there is an increased intensity of negative charge on central carbon of tertiary carbanion which further makes it unstable.
Vinyl carbanion stability.
Allylic 3º 2º 1º methyl alkenyl vinyl aryl electron poor electrophilic acidic carbon radicals.
Class structure stability pattern carbocations.
More importantly the bond angles between the vinyl cation carbons and the first carbon of the alkyl substituted was measured to be approximately 180 o.
The observation of enzyme catalyzed deuterium exchange via a stabilized carbanion provides convincing evidence for the decarboxylation of orotidine 5 monophosphate omp by ompdc to give the same carbanion intermediate.
But in any case the transition state leading to the phenyl carbanion will be more stable than the transition state leading to the vinyl carbanion because.
Allylic carbocations are able to share their burden of charge with a nearby group through resonance.
Absent π delocalization carbanions assume a trigonal pyramidal bent or linear geometry when the carbanionic carbon is bound to three e g methyl anion two e g phenyl anion or one e g acetylide anion substituents respectively.
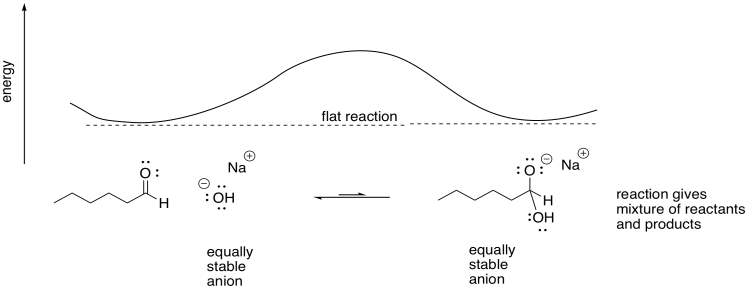
Factors that stabilize them will speed up reaction rates.
A vinyl carbocation has a positive charge on the same carbon as the double bond.
Certain carbanions also bond strongly to transition metals particularly those in low oxidation states through a combination of σ and π bonding with the ligand carbanion donating an unshared pair to form a σ bond or in the case of alkenes one or more pairs of π electrons to give dπ pπ bonding to the metal and receiving electron.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
The vinyl cation has an intense ir peak at 1987 cm 1 for the c c stretching.
Stability of vinyl cations.
It seems like a reasonable first approximation that they would both rehybridize similar amounts and the relaxed phenyl carbanion would remain more stable than the relaxed vinyl carbanion.
Stability of carbocation intermediates.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
Allylic 3º 2º 1º methyl alkenyl vinyl aryl electron poor electrophilic acidic carbanions.
This is very very unstable and ranks under a methyl carbocation in stability.
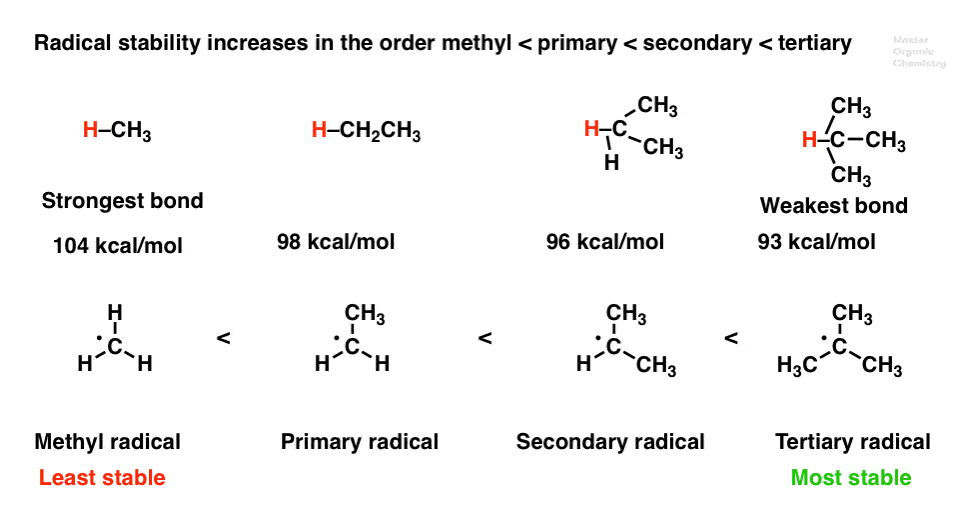
Thus it is very important to know their stability patterns.